Impact of Drug factors in dosage form design
- Solubility
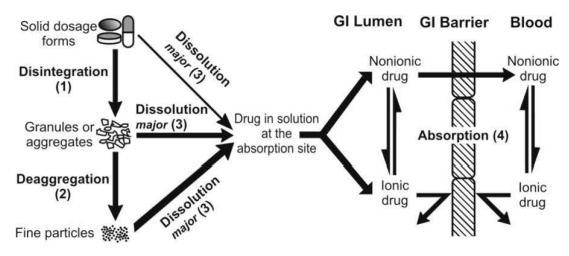
The solubility of a drug is a critical factor in dosage form design. If a drug is not soluble in water, it may be difficult to formulate into a dosage form that can be easily absorbed by the body. To overcome this problem, pharmaceutical companies may use solubilizing agents or formulate the drug in a lipid-based delivery system. Solubility also affects the choice of dosage form, as some forms such as tablets and capsules require the drug to be in a solid or crystalline form, whereas other forms such as solutions and suspensions require the drug to be in a dissolved or dispersed form.
- Stability
The stability of a drug is another important factor in dosage form design. Drugs can degrade over time due to factors such as light, heat, and humidity. Formulation scientists must ensure that the dosage form can protect the drug from degradation and maintain its potency throughout the shelf-life of the product. Various strategies such as using antioxidants or stabilizers can be employed to improve the stability of a drug in a dosage form.
- Bioavailability
Bioavailability refers to the fraction of the drug that reaches the systemic circulation after administration. Factors such as drug solubility, permeability, and formulation can affect the bioavailability of a drug. Dosage forms must be designed to optimize the bioavailability of the drug and ensure that it reaches the target site in the body. Controlled-release dosage forms can be designed to provide a sustained release of drug over a prolonged period of time, which can improve bioavailability and reduce the frequency of dosing.
- Dosing regimen
The dosing regimen refers to the frequency and duration of drug administration. Dosage forms must be designed to accommodate the dosing regimen and ensure that the drug is delivered to the body in the correct amount and at the correct intervals. For example, immediate-release dosage forms are designed to deliver the drug quickly and have a short duration of action, whereas extended-release dosage forms are designed to deliver the drug slowly and have a longer duration of action.
- Particle size and shape
The particle size and shape of a drug can influence the absorption, distribution, and bioavailability of the drug. If the drug particles are too large, they may not be absorbed efficiently, and if they are too small, they may be rapidly cleared from the body. Formulation scientists must consider the particle size and shape of the drug when designing dosage forms to ensure that the drug is delivered to the target site in the body.
- Chemical structure
The chemical structure of a drug can impact its solubility, stability, and bioavailability. For example, drugs with complex chemical structures may require specialized formulation techniques to ensure stability and effective delivery. Additionally, the chemical structure of a drug can affect its ability to cross cell membranes and reach its target site.
- Polymorphism
Polymorphism refers to the ability of a drug to exist in multiple crystal forms. Different crystal forms can have different properties, such as solubility and stability. Formulation scientists must carefully consider polymorphism when designing dosage forms to ensure that the drug is in the desired crystal form and remains stable throughout the shelf-life of the product.
- pH and ionization
The pH and ionization of a drug can affect its solubility and absorption. Formulation scientists must consider the pH and ionization of the drug when designing dosage forms to ensure that the drug is in the optimal form for absorption and bioavailability.
- Route of administration
The route of administration can also impact dosage form design. Different routes of administration, such as oral, topical, or parenteral (injection), require different types of dosage forms. For example, oral dosage forms need to be able to withstand the acidic environment of the stomach, while topical dosage forms need to be formulated to penetrate the skin barrier.
- Pharmacokinetics
Pharmacokinetics refers to the study of how a drug is absorbed, distributed, metabolized, and excreted by the body. Understanding a drug's pharmacokinetics is critical to designing a dosage form that can deliver the drug to the target site in the body. Formulation scientists must consider factors such as the drug's half-life, clearance rate, and volume of distribution when designing dosage forms.
- Toxicity
The toxicity of a drug is an important consideration in dosage form design. If a drug has a narrow therapeutic index or a high potential for toxicity, it may need to be formulated in a way that minimizes the risk of adverse effects. For example, sustained-release dosage forms can reduce the peak plasma concentration of a drug, which can help reduce toxicity.
- Manufacturing process
The manufacturing process can also impact dosage form design. The dosage form must be designed in a way that is compatible with the manufacturing process and can be produced on a large scale. Additionally, the dosage form must be stable during manufacturing and storage to ensure consistent quality and efficacy.
In conclusion, drug factors play a significant role in dosage form design. Pharmaceutical companies must take into account the unique physical and chemical properties of each drug and design dosage forms that optimize drug delivery, stability, bioavailability, and dosing regimen. By doing so, they can create safe and effective drugs that improve patient outcomes.


Great job on this post! Third-Party Manufacturing and PCD Pharma Franchise in India
ReplyDeleteLeading PCD Pharma and Medicine Manufacturer in West Kameng & Upper Subansiri, Arunachal Pradesh