PLASMA PROTEIN BINDING
- It is binding of drug to plasma protein in blood component. Binding of drugs to plasma proteins is reversible.
- Various plasma protein to which drug binds includes Albumin, α1-Acid glycoprotein (orosomucoid), lipoprotein, globulins, etc.
- Order of binding of drugs to various plasma proteins:
- Albumin > α1-Acid glycoprotein (orosomucoid) > Lipoproteins > Globulins
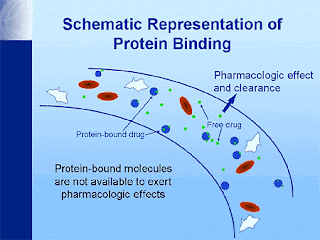
- Such bound drug is both pharmacokinetically as well as pharmacodynamically inert i.e. a protein bound drug is neither metabolized nor excreted nor it is pharmacologically active.
- A bound drug is also restricted since it remains confined to a particular tissue for which it has greater affinity. Moreover, such bound drug because of its enormous size cannot undergo membrane transport & thus its half life is increased.
- However this binding is rapidly reversible and non-specific – that is many drugs may bind to the same protein. Drug–plasma protein binding forms a "reservoir" of drug, but only the free (unbound) drug is available to the tissues to exert a therapeutic effect.
- Binding of drug generally is reversible process (hydrogen bond , hydrophobic bond ,ionic bond ,van der waal‘s forces ). Irreversible drug binding though rare (covalent binding) is often a reason for carcinogenicity or tissue toxicity of drug ;for example covalent binding of paracetamol metabolities to liver results in hepatotoxicity.
Binding of drugs falls into 2 classes:
1. Binding of drugs to blood components like-
A. Plasma proteins
B. Blood cells
2. Binding of drugs to extravascular tissue proteins, fats, bones ,etc
BINDING OF DRUG TO HUMAN SERUM ALBUMIN (HSA):
- Human serum albumin:
BINDING OF DRUG TO α1- ACID GLYCOPROTEIN (Α1-AGP OR AAG):
- Moloecular weight = 20,000-3,40,000
- Concentration = 0.04-0.4%
- Binds to basic drugs liKe imipramine, amitriptyline, nortriptyline, lidocaine, propranolol, quinidine, disopyramide, etc.
BINDING OF DRUGS TO LIPOPROTEINS
- Molecular weight = 20,000-3,40,000
- Concentration is variable
- Noncompetitive binding of drugs.
- Binding of drug occurs by dissolving in the lipid core of the protein Binds to Acidic (Diclofenac), Neutral (Cyclosporine A) and Basic (Chlrpromazine) drugs.
- More affinity to lipophilic drugs.
- Significant when level of human serum albumin and α1 -acid glycoprotein in plasma decreases.
BINDING OF DRUG TO GLOBULINS:
Type of
Globulin
|
Notes
|
α1 – globulin
[transcortin or CBG
(corticosteroid binding globulin)]
|
Binds
with steroidal drugs e.g. cortisone, prednisone, thyroxine, cyanocobalamine
|
α2–globulin (ceruloplasmin)
|
Binds with Vitamins: A, D,
E, K & cupric ions
|
β1-globulin (transferrin)
|
Binds with ferrous ions
|
β2 – globulin
|
Binds with carotenoids
|
γ – globulin
|
Binds specifically to
antigen
|
BINDING OF DRUG TO BLOOD CELLS:
Rate and extent of entry of drug into R.B.C. is more for lipophilic drugs. e.g.: Phenytoin.
Hemoglobin
|
Phenytoin,
Pentobarbital, Phenothiazine
|
Carbonic-anhydrase
|
Acetazolamide,
Chlorthiazide (carbonic anhydrase inhibitors)
|
Cell
membrane
|
Imipramine,
Chlorpromazine
|
FACTORS AFFECTING PROTEIN-DRUG BINDING:
1. RELATED TO DRUG :
A) Physicochemical Characteristics of Drugs:
- Protein binding is directly proportional to lipophilicity.
- Cloxacillin (More lipophilic - 95 % binding) > Ampicillin (Less lipophilic - 20 % binding)
B) Concentration of Drug in Body:
- Extents of binding depend on both the concentration of drug as well as protein.
- For the drug that binds with HSA, binding is not affected by drug Concentration. But for the drug that binds with AAG, binding is affected by drug concentration. eg. Lidocaine
C) Drug -Protein / Tissue Affinity:
- Lidocaine greater affinity for AAG than HSA.
- Digoxin has more affinity for proteins of cardiac muscles than those of skeletal muscles or plasma
- Iophenoxic acid great affinity for plasma protein that it has half life of 2.5 years.
2. RELATED TO PROTEINS:
A) Physicochemical Properties of Proteins:
- Lipoproteins and adipose tissue tends to bind with lipophilic drugs
- Physiologic pH determines presence of active anionic or cationic groups on the albumin molecules to bind a variety of drug.
B) Concentration of Plasma Proteins:
- Binding predominantly occurs with albumin as it is present in a higher concentration in comparison to other plasma proteins.
- Concentration of various proteins may change during diseased state that can alter extent of binding.
C) No. Of Binding Sites on the Protein:
- HSA has more binding sites than AAG.
- Drug may bind with more than one site on protein.eg. Indomethacin is known to bind 3 different sites.
- AAG is a protein with limited binding capacity because of its low concentration & low molecular size
3. DRUG INTERACTIONS
- When 2 or more drugs Can bind to same site , competition between them for interaction with binding site results .Such a drug-drug interaction for common binding site is called as DISPLACEMENT INTERACTION.
- Administration of phenylbutazone to a patient on a warfarin therapy results in displacement of latter from its binding site ,free warfarin cause the adverse hemorrahagic reactions which may be lethal
- The concentration & affinity for binding to site will determine the extent to which displacement will occur
- The drug with large Vd , redistributes into large volume of fluid & clinical effects may be insignificant. where as drug of small Vd ,remains confined to blood compartments shows serious toxic reactions.
EXAMPLE:
Assume that Drug A and Drug B are both protein-bound drugs. If Drug A is given, it will bind to the plasma proteins in the blood. If Drug B is also given, it can displace Drug A from the protein, thereby increasing Drug A's fraction unbound. This may increase the effects of Drug A, since only the unbound fraction may exhibit activity.
Before Displacement
|
After
Displacement
|
%
increase in unbound fraction
|
|
Drug A
|
|||
% bound
|
95
|
90
|
|
% unbound
|
5
|
10
|
+100
|
Drug B
|
|||
% bound
|
50
|
45
|
|
% unbound
|
50
|
55
|
+10
|
- For Drug A, the % increase in unbound fraction is 100%-- hence, Drug A's pharmacologic effect has doubled. This change in pharmacologic effect could have adverse consequences.
- This effect of protein binding is most significant with drugs that are highly protein-bound (>95%) and have a low therapeutic index, such as warfarin. A low therapeutic index indicates that there is a high risk of toxicity when using the drug. Since warfarin is an anticoagulant with a low therapeutic index, warfarin may cause bleeding if the correct degree of pharmacologic effect is not maintained. If a patient on warfarin takes another drug that displaces warfarin from plasma protein, it could result in an increased risk of bleeding.
4. Competition between Drugs and Normal Body Constituents:
- Bilirubin binding to HAS can be impaired by certain drugs & is of great concern in neonates whose BBB & Bilirubin metabolizing capacity are not efficient.
- Acidic drugs such as Sodium salicylate, sodium benzoate & sulfonamides displace bilirubin from its binding site. Free Bilirubin is no conjugated by the liver of neonates and thereby precipitates - KERNICTERUS‖ (characterized by degeneration of brain & mental retardation)
- It involves alteration of the protein structure by the drug thereby modifying its binding capacity e.g aspirin acetylates the lysine fraction of albumin thereby modifying its capacity to bind NSAIDS like phenylbutazone (increased affinity ) & flufenamic acid (decreased affinity).
- Physiological conditions:
- Species
- Age
- Pregnancy
- Ethnicity
- Gender
- Smoking
- Obesity
- Nutritional status
DISEASE
|
INFLUENCE ON PLASMA
PROTEINS
|
INFLUENCE ON PROTEINDRUG BINDING
|
Renal failure
(uremia)
|
Decreased albumin
content
|
Decreased
binding of acidic drugs. Neutral &
basic drugs are unaffected.
|
Hepatic failure
|
Decreased albumin
synthesis
|
Decreased
binding of acidic drugs. Binding of
basic drugs is normal or reduced depending upon α1 acid
glycoprotein levels
|
Inflammatory states (trauma, surgery, burns,
infections etc.)
|
Increased α1-acid glycoprotein levels
|
Increased
binding of basic drugs. Neutral & acidic drugs are unaffected.
|
METHOD OF DETERMINATION OF PLASMA PROTEIN BINDING:
1. EQUILIBRIUM DIALYSIS
2. ULTRAFILTRATION
3. ULTRACENTRIFUGATION
4. CHROMATOGRAPHIC METHODS
5. MICRODIALYSIS
- In addition to measuring the binding level of drug in plasma, these procedures examine the number of binding site, the affinity constant, and the nature of the protein involved.
- Allowing interpretation of the impact of binding on drug pharmacokinetics and response.
- The general procedure involves separation of free ligand (small molecules of drug) from bound species attached to protein.
- Two compartments are separated by membrane. At equilibrium 1st compartment contain plasma with protein and bound ligand. While free drug sequestered in buffer-solution compartment.
- Dialysis is carried for 4 hrs at 37°C has been found optimal for acidic & basic drugs.
- Unbound fraction drug = [D]
- [D]+ [DP]
- Where, [D] = drug conc. at buffer side & [D]+ [DP] = drug conc. in plasma and drug- protein complex.
- Results are influenced by:
- Drug properties,
- Protein (content & concentration),
- Volume of compartment
- Buffer strength and ionic composition, thickness & physiochemical characteristics of the membrane
- Volume shift if the drug takes vary long time than dilution or volume shift can occur due to concentration gradients.
- Adsorption of drug on membrane less for acidic & lipophilic drugs & more for lipophilic ,basic drugs
- Poorly water soluble drugs are difficult to study due to agglomeration and adherence to membrane.
 2. ULTRAFILTRATION:
2. ULTRAFILTRATION:- Separation of the protein and bound drug from free drug in solution occurs using a suitable membrane which retains the proteins and is assisted by positive pressure or centrifugation.
- Principal advantage : Speed (as little as 15 min)
- Disadvantages:
- Both equilibrium dialysis and Ultrafilration need radioisotopes for low
- Concentration or highly bound drug to provide sensitivity for quantization.
- Based on differential sedimentation of solutes, based on their molecular weights.
- High speed for long period (24h)
- Protein and bound drug are forced to the lower layer in the tube and the free drug is bound in the - middle cut.‖ & with any lipoprotein – bound drug near surface.
- Advantages:
- No membrane so no absorption of drug. Used for lipophilic drug.
- Tubes are made up of non-absorptive materials like nitrocellulose and polyallomer no adsorption & allowed the binding of water insoluble ,hydrophobic cyclosporine to be determined.
- Disadvantages:
- High cost
- Long period
- Cosedimentation of free and bound drug
- Possibility of disturbing the equilibrium
- Size exclusion gel permeation chromatography (slow & detection difficulty)
- HPLC (reduced time & extended scope of procedure)
- Disadvantage:
- Plugging up of the column due to high concentration of proteins.
- It is performed by perfusing of small diameter dialysis tubing with a carrier solution. small molecules in the sample , such as free drugs , diffuse in the fiber & are transported to collection vials for analysis .large molecules such as protein & drug-protein bound drugs are excluded by dialysis membrane. The dialysate can be analyzed by standard technique
- Microdialysis perfusion is rapid as ultrafiltration but the sample do not suffer from reequlibration during separation of free from bound drug
- Relative recovery = Dialysate conc.
- Surrounding conc.
- Relative recovery is the mean value of determinations before and after each experiment
- Unbound Fraction = (dialysate conc./ relative recovery)
- Plasma conc.
- It depends on
- The perfusion flow rate
- The diffusion characteristic of analyte
- The nature of matrix
- Properties and dimension of dialysis membrane.
- r = Ka * [D]
- Ka [D] +1 (Applied only when 1 binding site on protein + Protein-Drug complex is of 1:1 type)
- r = N*Ka*[D]
- Ka*[D] + 1 (Applied only when > 1 binding site on protein + Protein-Drug complex is other than 1:1 type)
- Where
- D = Free drug concentration
- Ka = Association rate constant
- r = number of moles of drug bound to total moles of proteins.
- Using above equation values of Ka and N can be obtained by plotting above equations in three different ways:
Importance of Protein or Tissue Binding of Drugs:
1. ABSORPTION:
- The absorption equilibrium is attained by transfer of free drug from site of administration into systemic circulation & when conc.
- However, binding of the absorbed drug to plasma protein decreases free drug concentration & disturbs such equilibrium. Thus, sink conditions & concentration gradient are re-established which act as a driving force for further absorption.
- This is particularly useful in case of ionized drugs which are transported with difficulty.
- Water insoluble drugs, neutral endogenous macromolecules such as heparin & several steroids & oil soluble vitamins are circulated & distributed to tissues by binding especially to lipoproteins which act as a vehicle for such hydrophobic compounds.
- 3. DISTRIBUTION
- Plasma protein binding restricts the entry of drugs that have specific affinity for certain tissues.
- This prevents accumulation of a large fraction of drug in such tissues & thus, subsequent toxic reactions.
- Plasma protein drug binding thus favors uniform distribution of drug throughout the body by its buffer function (maintains equilibrium between the free & the bound drug).
- A protein bound drug in particular does not cross BBB, the placental barrier & glomerulus.
- 4. TISSUE BINDING, APPARENT VOLUME OF DISTRIBUTION & DRUG STORAGE
- A drug that s extensively bound to blood components remain confined to blood .such a drug has a small volume of distribution & thus have small volume of distribution. A drug that shows extracellular tissue binding has a large volume of distribution.
- A tissue or blood component that has great affinity for a particular drug act as depot or storage site for that drug .e.g. RBC is storage site for lipophilic compound tetrahydrocannabinol
- The following equation derived shows that greater the unbound or free concentration of drug in plasma, larger its Vd
Vd = Vp + ( Vt fu)/fut
- Where
- Vd –apparent volume of distribution of drug
- Vp-volume of plasma
- Vt-volume of extracellular tissues
- fu - fraction of drug unbound in plasma
- fut - fraction of drug unbound to tissues
- Only the unbound or free drug is capable of being eliminated. This is because drug-protein complex cannot penetrate into metabolizing organ (liver).
- The large molecular size of the complex also prevents it from getting filtered through the glomerulus.
- Thus, drugs which are more than 95 % bound are eliminated slowly i.e. they have long elimination half-lives;
- For example – tetracycline which is only 65% bound has an elimination half life of 8.5 hours in comparison to 15.1 hours of doxycycline which is 93% bound to plasma proteins.
- However, penicillin have short elimination half-lives despite being extensively bound to plasma proteins .this is because rapid equilibrium occurs between free & bound drug & the free drug is equally rapidly excreted by active secretion in renal tubules
- 6. DISPLACEMENT INTERACTIONS & TOXICITY:
- More significant in case of drugs which are more than 95% bound .a displacement of just 1% of a 99% bound drug results in doubling of the free drug conc. i.e. a 100% rise. For a drug i.e. bound to a lesser extent e.g. 90 % ,displacement of 1 % results in only a 10 % rise in free drug conc.which may be insignificant clinically.
- Kernicterus in infants is an example of a disorder caused by displacement of bilirubin from albumin binding sites by the NSAIDS.
- With a drug of large Vd such as DIGOXIN, even a substantial increase in the degree of displacement of drug in plasma may not effect a large increase in free drug concentration & dose adjustment may not be required.
- Because:
- 1) only a small fraction of such a drug is present in plasma whereas most of it is localized in extravascular tissues,
- 2) following displacement the free drug ,because of its large Vd redistributes in a large pool of extravascular tissues.
- 7. DIAGNOSIS
- The chlorine atom of chloroquine when replaced with radio labeled I-131 can be used to visualize melanomas of the eye since chloroquine has a tendency to interact with melanin of eyes. The thyroid gland has a great affinity for iodine containing compounds, hence any disorder by tagging such compound with radioisotope of iodine.
- 8. THERAPY AND DRUG TARGETING:
- The binding of drugs to lipoproteins can be used for site specific delivery of hydrophilic moieties.
- This is particularly useful in cancer therapies since certain tumor cells have greater affinity for LDL than normal tissues. Thus binding of a suitable antineoplastic to it can be used as a therapeutic tool.
- HDL is similarly transported more to adrenal and testes .an example of site specific drug delivery in cancer treatment is that of estramustine.
- Estradiol binds selectively & strongly to prostrate & thus prostrate cancer can be treated by attaching nitrogen mustard to estradiol for targeting of prostrate gland. drug targeting prevents normal cells from getting destroyed.
- 9. DESIGN OF DOSAGE REGIMEN FROM PLASMA CONCENTRATION:
- If the therapeutic range, apparent Vd and clearance or half life of a drug is known, then dosage regimen can be designed to maintain drug concentration within the specified therapeutic range.
- Tmax = 3.32 t1/2 log (Cupper/C lower)
- Max maintanence dose = Vd/F (Cupper- C lower)
- After a convenient dosing interval t, smaller than tmax has been selected, the maintanence dose is given as :
Xo = (Xo max / tmax) t
Cssavg = (Cupper - C lower)
2.303 log (C upper /C lower)
Effect of protein binding on Drug Pharmacodynamic Effects:
For a drug showing little protein binding, the plasma acts simply as a watery solution in which the drug is dissolved. Where protein binding does occur, the behaviour of the drug may be influenced in several ways:
For a drug showing little protein binding, the plasma acts simply as a watery solution in which the drug is dissolved. Where protein binding does occur, the behaviour of the drug may be influenced in several ways:
- Extensive plasma protein binding will increase the amount of drug that has to be absorbed before effective therapeutic levels of unbound drug are reached. For example, acidic dugs (such as acetyl salicylic acid – aspirin) are often substantially bound to albumin.
- Elimination of a highly bound drug may be delayed. Since the concentration of free drug is low, drug elimination by metabolism and excretion may be delayed. This effect is responsible for prolonging the effect of the drug digoxin
- Changes in the concentration of plasma proteins will influence the effect of a highly bound drug. A low plasma protein level may occur in old age or malnutrition. It may also be caused by illness such as liver disease (remember that most plasma proteins are made in the liver), or chronic renal failure where there is excessive excretion of albumin. In each case the result is a smaller proportion of drug in bound form and more free drug in the plasma. The greater amount of free drug is able to produce a greater therapeutic effect and reduced drug dosages may be indicated in these cases
- Drugs may compete for binding with plasma proteins leading to interactions. This is significant for highly bound drugs such as the anticoagulant warfarin since even a small change in binding will greatly affect the amount of free drug. Such an effect is produced by the concurrent administration of aspirin, which displaces warfarin and increases the amount of free anticoagulant.
- Stereoselectivity was demonstrated for both albumin-binding sites 1 & 2.
- Stereoselective binding was reported for Ibuprofen enantiomers ,unbound fraction of R(-) Enantiomer (0.419 %)being significantly less than that S(+) enantiomer (0.643%).
- Stereoselective binding in humans was reported for acidic drugs such as etodolac,warfarin,pentobarbital,& for basic drugs like chloroquine,propanolol,methadone,etc
- Stereoselectivity in protein binding of enantiomer can also differ between species . for propanolol ,a basic drug bound to AAG ,R-enantiomer binds less than S-isomer in humans & dogs,the reverse is observed in rats
- A difference in the binding of 2 isomers , Quinine & Quinidine , fu was 7.5 & 12.3 % respectively .
- State importance of absorption rate constant.
- Importance of elimination half life
- Suppose volume of distribution of a particular drug is more, then what does that it mean?
- How can you design an effective dosage regimen based on plasma concentrations?
- What are the factors affecting renal clearance?
- What is protein binding ? Describe binding of drug with various proteins.
- State consequences of high plasma protein binding
- Describe the methods of determination of protein binding.
- Describe factors affecting protein binding
- How protein binding affects the Pharmacodynamic characteristic of drug.
- Explain the significance of drug-protein binding
- Applied Biopharmaceutics & Pharmacokinetics fourth edition: Leon Shargel, Andrew B.C. YU.
- Biopharmaceutics & Pharmacokinetics: a treatise: D. M. Brahmankar, S. B. Jaiswal
- Biopharmaceutics and Clinical Pharmaceutics by Robert Notari, p.g. 290-333,48-98
- Textbook of Biopharmaceutics and Pharmacokinetics by Dr Shobha Rani, p.g 84—205
- Gibaldi, M. 1984 "Biopharmaceutics and Clinical Pharmacokinetics", 3rd ed., Lea & Febiger, Chapter 12, page 214.
- Rowland and Tozer, Ch 3, 10, 19 (From an Internet Article)
- IUPAC Compendium of Chemical Terminology 2nd Edition (1997)
- J Pharm Sci 88:292-302, 2000 Encyclopedia volume – 13
- www.nottingham.ac.uk/nursing/sonet/rlos/bioproc
- www.ricerca.com/pages/virtua
- www.medscape.com
- www.wikipedia.com
- www.ncbi.nlm.nih.gov
THANK YOU ... !!!







Great post! I really appreciate how clearly you explained the connection between diet and pancreatic cancer. Many people underestimate the role of nutrition in prevention and recovery, so it’s refreshing to see a detailed guide like this. The section about antioxidant-rich foods was especially helpful. Looking forward to reading more insightful content from you.protein powder for cancer patients
ReplyDeleteNice articles and your information valuable and good articles thank for the sharing information Novel Proteins for Sensitive Dogs
ReplyDelete