PLASMA PROTEIN BINDING
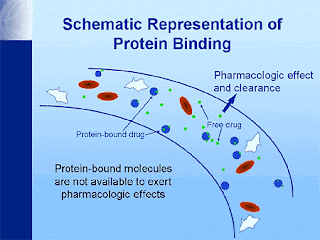
Introduction: It is binding of drug to plasma protein in blood component. Binding of drugs to plasma proteins is reversible. Various plasma protein to which drug binds includes Albumin, α1-Acid glycoprotein (orosomucoid), lipoprotein, globulins, etc. Order of binding of drugs to various plasma proteins: Albumin > α1-Acid glycoprotein (orosomucoid) > Lipoproteins > Globulins Such bound drug is both pharmacokinetically as well as pharmacodynamically inert i.e. a protein bound drug is neither metabolized nor excreted nor it is pharmacologically active. A bound drug is also restricted since it remains confined to a particular tissue for which it has greater affinity. Moreover, such bound drug because of its enormous size cannot undergo membrane transport & thus its half life is increased. However this binding is rapidly reversible and non-specific – that is many drugs may bind to the same protein. Drug–plasma protein binding forms a "reservoir" o