BIOEQUIVALENCE: Its type and method of studying bioequivalence

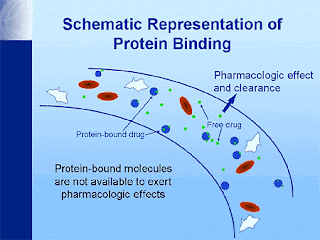
BIOEQUIVALENCE: It‘s commonly observed that there are several formulations of the same drug, in the same dose, in similar dosage form and meant to be given by the same route. in order to ensure clinical performance of such drug products, bioequivalence studies should be performed. TYPES OF EQUIVALENCE Chemical Equivalence: When 2 or more drug products contain the same labeled chemical substance as an active ingredient in the same amount. Pharmaceutical Equivalence: When two or more drug products are identical in strength, quality, purity, content uniformity, disintegration and dissolution characteristics; they may however differ in excipients. Bioequivalence: A relative term which denotes that the drug substance in two or more dosage forms, reaches the systemic circulation at the same relative rate and to the same relative extent i.e., their plasma concentration time profiles will be identical without significant statistic...