Effect of Micromeritics Property on Preformulation: Particle Size, Particle Shape & Density
1. Particle
Size:
·
Characterized
using these term:
o
Very
coarse (#8)
o
Coarse
(#20)
o
Moderately
coarse (#40)
o
Fine
(#60)
o
Very
fine (#80)
·
Size of
the particles may be expressed as follows:
o
Surface
diameter, ds
o
Volume
diameter, dv
o
Projected
diameter, dp
o
Stokes
diameter, dst
o
Sieve
diameter, dsieve
o
Volume
– surface diameter, dvs – same volume to surface area ratio
|
Table 1.1: Common Techniques
for Measuring Fine Particles of Various Sizes
|
|
|
Technique
|
Particle size
(micro meters)
|
|
Microscopic
|
1-100
|
|
Sieve
|
>50
|
|
Sedimentation
|
>1
|
|
Elutriation
|
1-50
|
|
Centrifugal
|
<50
|
|
Light scattering
|
0.5 - 50
|
Methods to determine particle size:
·
Sieving:
o
Simple
& inexpensive
o
Dry
powder require
·
Microscopy:
o
Particle
size determine by calibrated grid background
o
Calibration
factore:
o
(No. of
division of stage micrometer/No. of division of eye-piece micrometer)*100
o
Slow and
tedious method
·
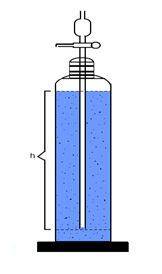
Sedimentation
method:
o
Particle
size calculated by stokes law
o
o
Where,
§
h =
distance fall in time, t
§
no =
viscosity of medium
§
ps =
density of particles
§
po =
density of dispersion medium
§
g =
gravitational acceleration
§
t =
time
·
Light
scattering:
o
Particle
size determine by the diversion of light pathway that reaching the sensor.
o
Quick
and fast
Particle size effect on following:
·
Formulation:
o
Uniform
particle size and shape have good flow properties
o
Uniform
size particle produce good & uniform dispersion
o
Uniform
size produce good compaction during tablet manufacturing
§
Defects
in tablets have less chances
o
Size
alsoplays a role in the homogeneity of the final tablet.
o
Large
differences in size exist between the active components and excipients, de-mixing
effects can occur.
·
Bioavailability
o
Uniform
and smaller size particles have good absorption and therefore good
bioavailability.
o
For
example, the bioavailability ofgriseofulvin and phenacetin is directly related
tothe particle size distributions of these drug
o
It is
now generally recognized that poorly soluble drugs showing a dissolution rate-
limiting step in the absorption process will be more readily bioavailable
when administered in a finely subdivided
state than as a coarse material
·
Stability:
o
Smaller
particle (in colloidal range) improve stability of dispersion
o
Bcz,
§
Brownian
motion observed
§
Therefore
sedimentation is law
2. Particle
Shape:
·
Formulation:
o
Sphere
particle have good flow property compare to other
o
Irregular
shape particle have not good flow property because they cloge during movement
o
Packing
and compaction property depend on particle shape
o
Parenteral
formulation have only sphere particles
·
Bioavailability:
o
Surface
area à Dissolution à absorption
o
Surface
area also depend on shape of particle
o
Increase
surface area à increase bioavailability
·
Stability:
o
Sphere
particle take more time to stable compare to other shape of particle
3.
Density:
The ratio of mass to volume is known as density
·
Types of density:
o
Bulk
density:
§ Bulk drug
powder is sieved through 40 mesh screen. Weight is taken and poured into a
graduated cylinder via a large funnel. The volume is called bulk volume.
o
Tapped
density:
§
Bulk
powder is sieved through 40 mesh screen.
§
Weight
is taken and poured into a graduated cylinder.
§
The
cylinder is tapped 1000 times on a mechanical tapper apparatus.
§
The
volume reached a minimum – called tapped volume.
o
True
density: It actual density of the solid material.
§
Solvents
of varying densities are selected in which the powder sample is insoluble.
§
Small
quantity of surfactant may be mixed with the solvent mixture to enhance wetting
and pore penetration.
§
After
vigorous agitation, the samples left to stand undisturbed until floatation or
settling has reached equilibrium.
§
The density of that solvent is determined
accurately with a pycnometer.
o
Granule
density:
§
may
affect compressibility, tablet porosity, disintegration, dissolution
·
Source of variation of bulk density:
o
Method
of crystallization, milling, formulation.
Significance:
·
Bulk
density:
o
Bulk
density is required during the selection of capsule size for a high dose drug.
o
In case
of low dose drug mixing with excipients is a problem if the bulk densities of
the drug and excipients have large difference.
·
Tapped
density:
o
Knowing
the dose and tapped density of the formulation, the capsule size can be
determined.
·
True
density:
o
From
bulk density and true density of powder,
the void volume or porosity can be measured.



Comments
Post a Comment