Dissolution Apparatus 3 & 4 (Reciprocating cylinder & Flow through cells)
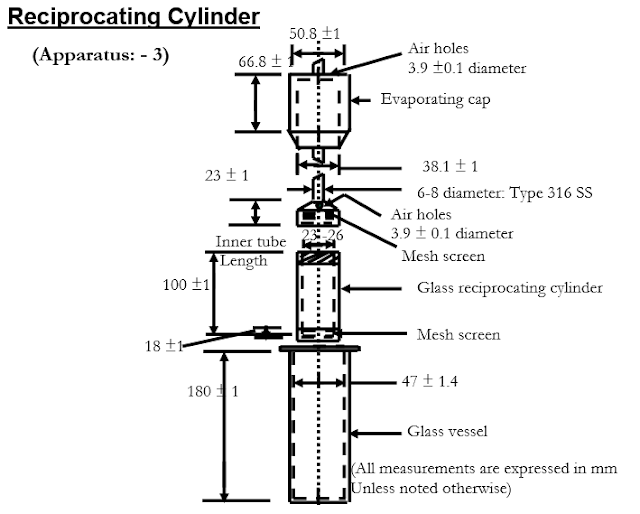
Apparatus: - 3 (Reciprocating Cylinder)
Apparatus:-
Consists of a set of set of cylindrical, flat
bottomed glass vessels; a set of glass reciprocating cylinders; SS fittings
& screen that are made of suitable material; and a motor & drive
assembly to reciprocate the cylinders.
No part of the assembly, including the environment
in which the assembly is placed, contributes significant motion, agitation or
vibration beyond that due to the smooth, vertically reciprocating cylinders.
Reciprocation should be at the tolerance of ± 5% at
a distance of 9.9 to 10.1 cm.
Apparatus
suitability test:-
Reference
standards:-
- USP Chlorpheniramine extended release tablets RS (For single unit drug release)
- USP Theophylline extended release beads RS (For multiple units).
Dissolution Medium:-Proceed as directed earlier.
Procedure:-
- Place the stated volume of dissolution medium in each vessel of the apparatus.
- Assemble & equilibrate the medium to 37 ±0.5°, and remove the thermometer.
- Place one dosage form in each cylinder and operate the apparatus.
- When capsule shell interfere with the analysis, remove the content of not less than 6 capsules as completely as possible, and dissolve the empty capsule shell in the specified volume of the dissolution medium.
Time and Interpretation: - Proceed as directed earlier.
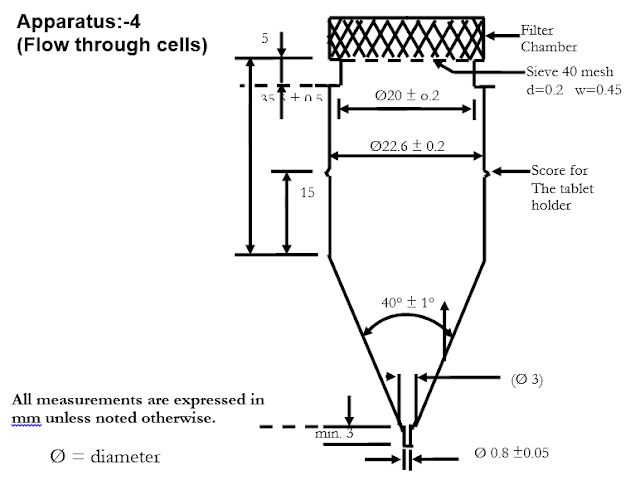
Apparatus:-4 (Flow through cells)
Apparatus:-
Apparatus
consists of: reservoir, pump, a flow
through cell, water bath.
The cell size specified in the individual monograph.
Pump delivery—240 & 960ml/hr with standard flow
rates of 4,8,16 ml per minute.
Flow must be volumetric and sinusoidal at a
pulsation rate of 120 + 10 pulses per minute. Standard cell diameter are
12 & 22.6 mm, the bottom cone is
filled with small glass beads of about 1 mm. diameter with one bead of about 5
mm. positioned at the apex to protect the fluid entry tube.
- A Tablet holder.
- O Rings for
fixation of apparatus.
The cell is immersed in medium& temp is
maintained at 37±0.5°
Apparatus
suitability & Dissolution medium:-
As directed under apparatus
1 & 2.
Procedure:-
- Place glass beads in to the cell & 1 dosage form unit on top of the beads or on a wire carrier.
- Introduce the dissolution medium through the bottom of the cell to obtain specified floe rate with an accuracy of 5%.
- When capsule shell interfere with the analysis, remove the content of not less than 6 capsules as completely as possible, and dissolve the empty capsule shell in the specified volume of the dissolution medium.
- Make any necessary correction. Correction factor greater than 25% of the labeled content is unacceptable.
Time & Interpretation: - Proceed as directed under apparatus 1 & 2.
Flow
through cell (E. P.).
Procedure:-
- Place one unit of the preparation to be examined in chamber A. Close the cell with the prepared filtered assembly.
- Heat the dissolution medium to an appropriate temp. By considering M.P. point of the preparation.
- Using pump, introduce warm dissolution medium through the bottom of the cell to obtain a suitable continuous flow through an open or closed circuit at the prescribed rate.
- The preparation spreads through the dissolution medium according to its physico-chemical properties. Large volume suppositories may be tested.
Delayed
Release (Enteric coated) articles.
Use method A or B and the apparatus specified in the
individual monograph.
Apparatus
suitability test: - As directed earlier.
METHOD: -
A
Procedure:-
- Acid Stage: - Place 750 ml of 0.1N HCl in the vessel. Operate the apparatus for 2 hrs. And withdraw sample.
- Continue testing through all the levels unless the results of both acid and buffer stages conform at an earlier level.
- Quantity of the active ingredient dissolved from the units tested confirm to Acceptance table given below.
|
Level
|
No. Tested
|
Criteria
|
|
A1
|
6
|
No individual value exceeds 10% dissolved
|
|
A2
|
6
|
Average of the 12 units (A1+A2) is not more than 10 % dissolved, no
individual unit is greater than 25 % dissolved
|
|
A3
|
12
|
Average of the 24 units (A1+A2+A3) is not more than 10% dissolved, and
no individual unit is greater than 25% dissolved.
|
Method B:-
- Acid Stage: 1000ml 0.1 N HCL and operate for 2hrs.
- Buffer Stage: Use buffer that previously has been equilibrated to a temp. of 370+0.50
- Add 1000ml of pH 6.8+0.05 phosphate buffer, prepared by mixing 0.1N HCL with 0.02M tribasic sodium phosphate (3:1)
- Operate for 45 min.
Interpretation:
- Proceed as directed in Method A







Comments
Post a Comment