BASIC CONCEPTS AND IMPORTANCE OF VARIOUS PHARMACOKINETIC PARAMETERS
INTRODUCTION:
Pharmacokinetics, sometimes abbreviated as PK, (from Ancient Greek pharmakon "drug" and kinetikos "to do with motion") is dedicated to the determination of the fate of substances administered externally to a living organism. Pharmacokinetics is the science of the kinetics of drug absorption, distribution, and elimination (ie, excretion and metabolism). The description of drug distribution and elimination is often termed drug disposition.
Pharmacokinetics is often studied in conjunction with pharmacodynamics.
Pharmacodynamics explores what a drug does to the body, whereas pharmacokinetics explores what the body does to the drug. Pharmacokinetics includes the study of the mechanisms of absorption and distribution of an administered drug, the rate at which a drug action begins and the duration of the effect, the chemical changes of the substance in the body (e.g. by enzymes) and the effects and routes of excretion of the metabolites of the drug.
Pharmacokinetics is divided into several areas which includes the extent and rate of Absorption, Distribution, Metabolism and Excretion. This is commonly referred to as the ADME scheme. However recent understanding about the drug-body interactions brought about the inclusion of new term Liberation.
Now Pharmacokinetics can be better described as LADME.
- Liberation is the process of release of drug from the formulation.
- Absorption is the process of a substance entering the body.
- Distribution is the dispersion or dissemination of substances throughout the fluids and tissues of the body.
- Metabolism is the irreversible transformation of parent compounds into daughter metabolites.
- Excretion is the elimination of the substances from the body. In rare cases, some drugs irreversibly accumulate in a tissue in the body.
Pharmacokinetics describes how the body affects a specific drug after administration. Pharmacokinetic properties of drugs may be affected by elements such as the site of administration and the concentration in which the drug is administered. These may affect the absorption rate.
To study these phases many pharmacokinetic parameters are required. Few of them are discussed here.
1. BIOLOGICAL HALF LIFE (t1/2) : (Synonym: Elimination half life)
The biological half-life or elimination half life of a substance is the time it takes for a substance (drug, radioactive nuclide, or other) to lose half of its pharmacologic, physiologic, or radiologic activity. In a medical context, half-life may also describe the time it takes for the blood plasma concentration of a substance to halve ("plasma half-life") of its steady-state. The relationship between the biological and plasma half-lives of a substance can be complex, due to factors including accumulation in tissues, active metabolites, and receptor interactions.
EXAMPLES:
Substance (Half-life)
- Amiodarone (25 days)
- Cisplatin (30 to 100 h)
- Chlorambucil (1.53 h)
- Digoxin (24 to 36 h)
- Fluoxetine (1 to 6 days)
- The active metabolite of fluoxetine is lipophilic and migrates slowly from the brain to the blood. The metabolite has a biological half-life of 4 to 16 days.
- Methadone (15 to 60 h)
- In rare cases up to 190 h.
- Oxaliplatin (14 min.)
- Salbutamol (7 h )
EQUATION:
- t1/2 = 0.693/Ke Where Ke = Elimination rate constant
- This equation holds true for first order kinetics.
- Value of Ke can be obtained by plotting graph of log C v/s time.
- With Recent advances in pharmacokinetics it is now found that t1/2 is secondary parameter which depends on primary parameters like clearance and volume of distribution.
- t1/2 = 0.693 * Vd/CLT
Importance of Biological half life:
- Adjustment of doses can be made by use of biological half life.
- Dosing regimen can be decided from biological half life.
- It is of very much importance in toxicity studies.
2. ABSORPTION RATE CONSTANT:
Introduction:
- Expressed by Ka
- The rate of drug absorption can be zero order, first order, pseudo zero order, pseudo first order, etc.
- Generally for I.R dosage form Ka is first order because of physical nature of drug diffusion.
- For I.V. infusion and certain controlled-release drug products, Ka will be zero-order rate constant.
- Ka determined by:
- Method of residuals
- Flip-Flop method of Ka and KE
- Wagner – Nelson Method
- Loo – Riegelman method
Importance:
- Designing a multiple-dosage regimen
- Ka and Ke helps to predict peak and trough plasma drug concentrations following multiple dosing.
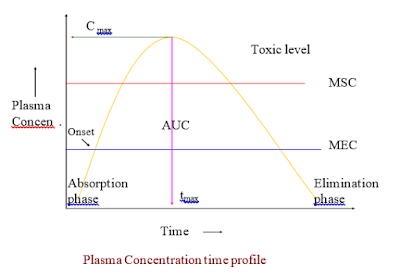
- In bioequivalence studies, drug products are given in chemically equivalent (i.e. pharmaceutical equivalent) doses. The respective rates of systemic absorption may not differ markedly. For these studies, tmax or time of peak drug concentration can be very useful in comparing the respective rates of absorption of a drug from chemically equivalent drug products.
3. ELIMINATION RATE CONSTANT (Ke)
Introduction:
- Ke is summation of rate constants for each process like urinary excretion, metabolism, biliary excretion, pulmonary excretion, etc.
- KE = Km + Kb + Kl +..........
- FOR, Zero order rate of elimination is constant irrespective of plasma concentration:
- Er = Ke.
- FOR, First order: rate of elimination proportional to plasma concentration. Constant Fraction of drug eliminated per unit time.
- Er = dC/dt = - Ke C
IMPORTANCE OF ELIMINATION RATE CONSTANT:
- Determination of Ke is important for selection of dose regimen.
- Also in dose adjustment in renal impairment.
4. VOLUME OF DISTRIBUTION
- The volume of distribution (VD) , also known as apparent volume of distribution, is used to quantify the distribution of a medication between plasma and the rest of the body after oral or parenteral dosing.
- It is defined as the volume in which the amount of drug would need to be uniformly distributed to produce the observed blood concentration.
- Volume of distribution may be increased by renal failure (due to fluid retention) and liver failure (due to altered body fluid and plasma protein binding). Conversely it may be decreased in dehydration.
- The volume of distribution is given by the above equation:
- Therefore the dose required to give a certain plasma concentration can be determined if the VD for that drug is known.
- The VD is not a physiologic value; it is more a reflection of how a drug will distribute Throughout the body depending on several physicochemical properties, e.g. solubility, charge, size, etc.
- The units for Volume of Distribution are typically reported in (ml or liter)/kg body weight.
- The fact that VD is a ratio of a theoretical volume to a fixed unit of body weight explains why the VD for children is typically higher than that for adults, even though children are smaller and weigh less. As body composition changes with age, VD decreases.
- EXAMPLES: Drug,VD,Comments
- Warfarin: 8L, Reflects a high degree of plasma protein binding.
- Theophylline, Ethanol : 30L, Represents distribution in total body water.
- Chloroquine : 15000L, Shows highly lipophilic molecules which sequester into total body fat.
- NXY-059 : 8L, Highly-charged hydrophilic molecule.
- Vd rarely corresponds to a real volume & thus also called APPARENT volume of distribution….
- Approx physiological volumes for a 70 kg man:
- Plasma~3 L
- ECF ~ 16 L
- TBW ~ 42 L
- Click here to see more
5. RENAL CLEARANCE :
Definition:
- It is the volume of blood from which the drug is totally removed in unit time through renal excretion.
- Expressed as CLR
- It has units : mL/min
- Major Organ for Excretion of Drugs is the Kidney.
- Functional Units are
- Nephron
- Bowman‘s Capsule
- Proximal Tubule
- Loop of Henle
- Distal Tubule
- Collecting Duct
- CLR = Rate of urinary excretion / Plasma drug concentration
- Physiologically,
- CLR = Rate of Filtration + Rate of Secretion - Rate of Reabsorption
- Plasma drug concentration (C)
- Click here for detail study
6. TOTAL CLEARANCE : (Synonym: Total systemic clearance)
Definition:
It is the volume of plasma completely cleared of drug per unit time by all routes and mechanisms. It is the sum of all the individual clearances by all the eliminating organs. i.e. It is the sum of all the individual clearances by all the eliminating organs…
CLT = CLR + CLH + CLP + …
- Where,
- CLR = Renal clearance,
- CLH =Hepatic clearance,
- CLP =Pulmonary clearance etc.
- Expressed in terms of ml/min.
- Click here for detail study on Total clearance
Introduction:
It is binding of drug to plasma protein in blood component. Binding of drugs to plasma proteins is reversible.
Various plasma protein to which drug binds includes
- Albumin, α1-Acid glycoprotein (orosomucoid), lipoprotein, globulins, etc.




Comments
Post a Comment